Immunoparasitology
TEL +81-6-6879-8333
FAX +81-6-6879-8332
Overview
In our immunoparasitology laboratory, we use the apicomplexan protozoan parasite Toxoplasma gondii as a model for exploring host defense systems and pathogenesis. Our research goal is to elucidate the molecular mechanisms underlying the interface between the host and pathogen.Antiviral host factors and their applicationto a cure for HIV infection.
Toxoplasma gondii is an obligatory intracellular protozoan pathogen that causes lethal toxoplasmosis in humans and animals. One third of the global population is thought to be infected with this pathogen, making it the “most successful parasite.” T. gondii infects virtually all nucleated cells in warm-blooded animals. The parasite forms a special membranous structure called a “parasitophorous vacuole (PV).” The host-parasite interaction takes place through the PV. In response to T. gondii, the host immune system produces inflammatory cytokines such as interleukins, chemokines, and interferons. Interferon-γ (IFN-γ) is the most important host factor for inducing anti-T. gondii responses, which suppress and kill the parasites. One of the main projects in our laboratory is to identify the IFN-γ-induced anti-T. gondii host defense mechanisms involved in innate and adaptive immunity. Recently, we found that IFN-γ-inducible GTPases called GBPs are important for T. gondii PV disruption, and that their function in anti-T. gondii responses requires autophagy proteins; this suggests an unexpected link between IFN-γ-induced immunity and autophagic pathways. On the other hand, virulent T. gondii suppress IFN-γ-induced host immunity and even manipulate host immune cells to maximize the virulence of the parasite. Another main project in our laboratory is to identify novel virulence mechanisms used by T. gondii. For example, we recently showed that a T. gondii-secreting virulence factor, GRA6, directly activates the host transcription factor NFAT4 to induce chemokines and recruit neutrophils to eradicate the parasite. Thus, our laboratory is focusing on host-parasite interactions via immunoparasitological mechanisms.
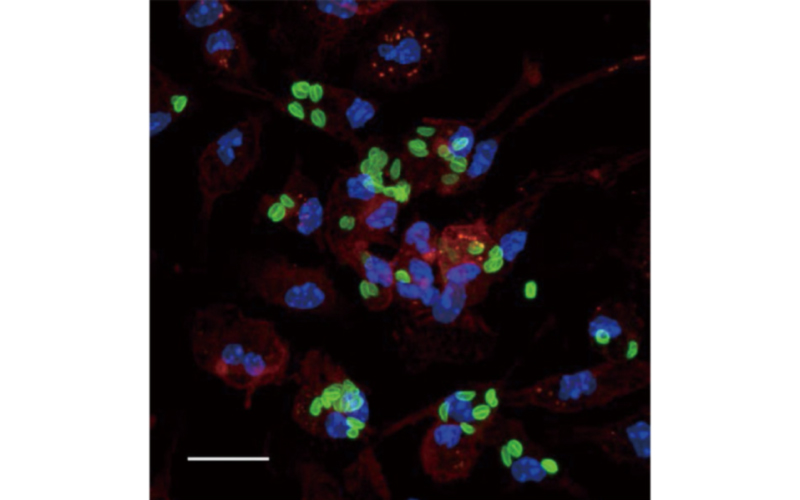
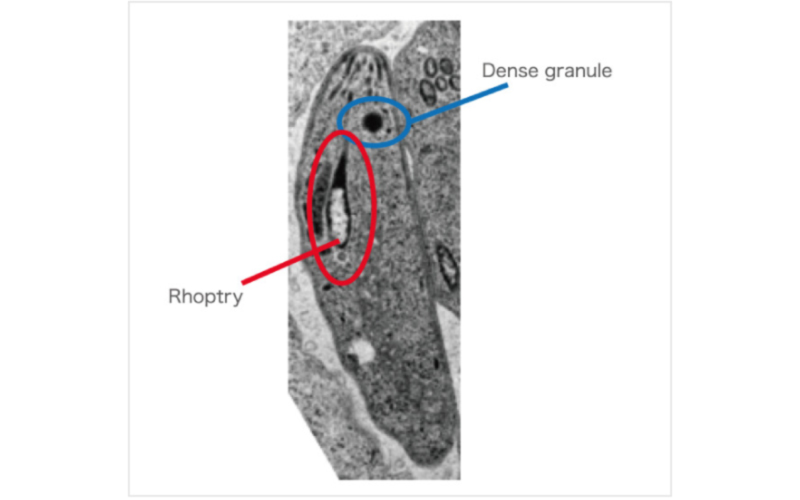
Pathogenic proteins are secreted from Dense granules and Rhoptry.
Principal Investigator
Masahiro Yamamoto Professor
Research field
Education history
| 2001 | Graduated from undergraduate course of Faculty of Science, The University of Tokyo |
|---|---|
| 2003 | Graduated from master course of graduate school of medicine, Osaka University |
| 2006 | Graduated from Ph.D course of graduate school of medicine, Osaka University |
Research and career history
| 2003 | RIKEN, the Junior Research Associate |
|---|---|
| 2006 | Research fellow, the Japan Society for the Promotion of Science |
| 2006 | Post doctoral fellow, Research Institute for Microbial Diseases, Osaka University |
| 2006 | Assistant Professor, Department of Immune regulation, Graduate School of Medicine, Osaka University |
| 2010 | Associate Professor, Department of Immune regulation, Graduate School of Medicine, Osaka University (-2011) |
| 2012 | Associate Professor, Department of Immunoparasitology Research Institute for Microbial Diseases, Osaka University |
| 2012 | Associate Professor, Department of Immunoparasitology, WPI Immunology Frontier Research Center, Osaka University |
| 2013 | Professor, Department of Immunoparasitology Research Institute for Microbial Diseases, Osaka University Professor, Department of Immunoparasitology, WPI Immunology Frontier Research Center, Osaka University |
| 2018 | Distinguished Professors, Osaka University |
Prize
| 2006 | Yamamura Prize |
|---|---|
| 2007 | JSI Young Investigator Award |
| 2011 | The Commendation for Science and Technology by the MEXT (The Young Scientists' Prize) |
| 2018 | The Japan Medical R&D Grand Prize |
| 2018 | The JSPS Prize 2018 |
Members
- Masahiro Yamamoto Professor
myamamotobiken.osaka-u.ac.jp - Myoung Ho Jang Guest Associate Professor
- Miwa Sasai Associate Professor
m-sasaibiken.osaka-u.ac.jp - Ma Jisu Assistant Professor
majisubiken.osaka-u.ac.jp - Mari Enomoto Laboratory Technician
enomoto-mifrec.osaka-u.ac.jp
Achievements
Publications
- Bando H, Lee Y, Sakaguchi N, Pradipta A, Ma JS, Tanaka S, Cai Y, Liu J, Shen J, Nishikawa Y, Sasai M, Yamamoto M. Inducible Nitric Oxide Synthase Is a Key Host Factor for Toxoplasma GRA15-Dependent Disruption of the Gamma Interferon-Induced Antiparasitic Human Response. MBio. (2018) 9(5).
- Bando H, Sakaguchi N, Lee Y, Pradipta A, Ma JS, Tanaka S, Lai DH, Liu J, Lun ZR, Nishikawa Y, Sasai M, Yamamoto M. Toxoplasma Effector TgIST Targets Host IDO1 to Antagonize the IFN-γ-Induced Anti-parasitic Response in Human Cells. Front Immunol. (2018) 9:2073.
- Sasai M, Sakaguchi N, Ma JS, Nakamura S, Kawabata T, Bando H, Lee Y, Saitoh T, Akira S, Iwasaki A, Standley DM, Yoshimori T, Yamamoto M. Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense. Nature Immunology. (2017) 18:899-910.
- Lee Y, Sasai M, Ma J, Sakaguchi N, Ohshima J, Bando H, Saitoh T, Akira S, Yamamoto M. p62 plays a specific role in interferon-γ-induced presentation of a Toxoplasma vacuolar antigen. Cell Reports. (2015) 13:223-233.
- Ohshima J, Sasai M, Liu J, Yamashita K, Ma JS, Lee Y, Bando H, Howard JC, Ebisu S, Hayashi M, Takeda K, Standley DM, Frickel EM, Yamamoto M. RabGDIα is a negative regulator of interferon-γ-inducible GTPase-dependent cell-autonomous immunity to Toxoplasma gondii. Proc Natl Acad Sci U S A. (2015) 112:E4581-4590.
- Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, Takeda K, Yamamoto M. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med. (2014) 211:2013-2032.
- Ohshima J, Lee Y, Sasai M, Saitoh T, Ma JS, Kamiyama N, Matsuura Y, Pann-Ghill S, Hayashi M, Ebisu S, Takeda K, Akira S, Yamamoto M . Role of the mouse and human autophagy proteins in IFN-Γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. (2014) 192: 3328-3335.
- Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DS, Soldati-Favre D, Horie K, Takeda J, Takeda K. A cluster of interferon-Γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity (2012) 37:302-313.
- Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, Kimura T, Okamoto T, Okuyama M, Kayama H, Nagamune K, Takashima S, Matsuura Y, Soldati-Favre D, Takeda K. ATF6b is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. (2011) 208:1533-1546.
- Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, Soldati-Favre D, Takeda K. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3.J Exp Med. (2009) 206: 2747-2760.
- Yamamoto M, Uematsu S, Okamoto T, Matsuura Y, Sato S, Kumar H, Satoh T, Saitoh T, Takeda K, Ishii KJ, Takeuchi O, Kawai T, Akira S. Enhanced TLR-mediated NF-IL6 dependent gene expression by Trib1 deficiency. J Exp Med. (2007) 204:2233-9.
- Yamamoto M, Sato S, Saitoh T, Sakurai H, Uematsu S, Kawai T, Ishii KJ, Takeuchi O, Akira S. Cutting Edge: Pivotal Function of Ubc13 in Thymocyte TCR Signaling. J Immunol. (2006) 177:7520-4.
- Yamamoto M, Okamoto T, Takeda K, Sato S, Sanjo H, Uematsu S, Saitoh T, Yamamoto N, Sakurai H, Ishii KJ, Yamaoka S, Kawai T, Matsuura Y, Takeuchi O, Akira S. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol. (2006) 7:962-70.
- Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, Saitoh T, Yamaoka S, Yamamoto N, Yamamoto S, Muta T, Takeda K, Akira S. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkBz. Nature. (2004) 430:218-22.
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. (2003) 4:1144-50.
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of Adaptor TRIF in the MyD88-Independent Toll-Like Receptor Signaling Pathway. Science. (2003) 301:640-3.
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-b Promoter in the Toll-Like Receptor Signaling. J Immunol. (2002) 169:6668-72.
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature.(2002) 420:324-329.
