Cellular Immunotherapy
TEL +81-6-6879-3871
FAX +81-6-6879-3879
Overview
Identification of cancer-specific cell surface antigens and development of CAR-T cell therapy targeting them
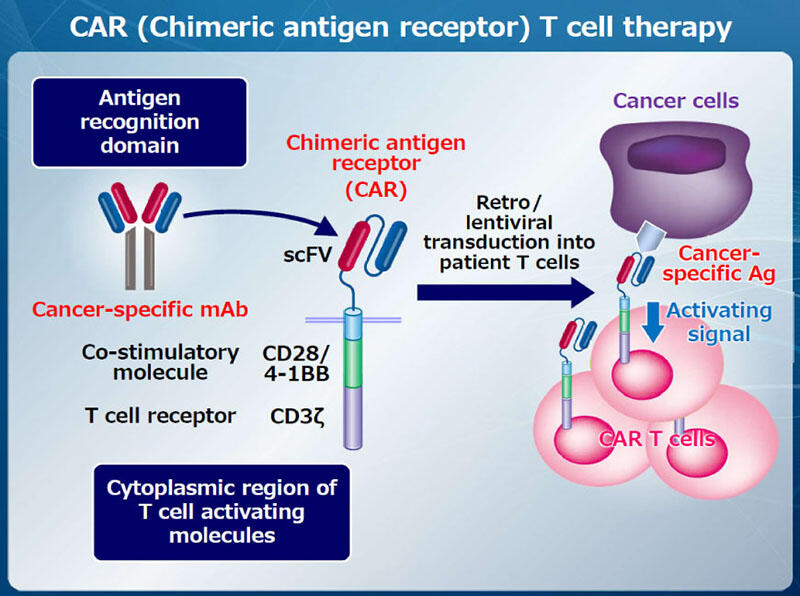
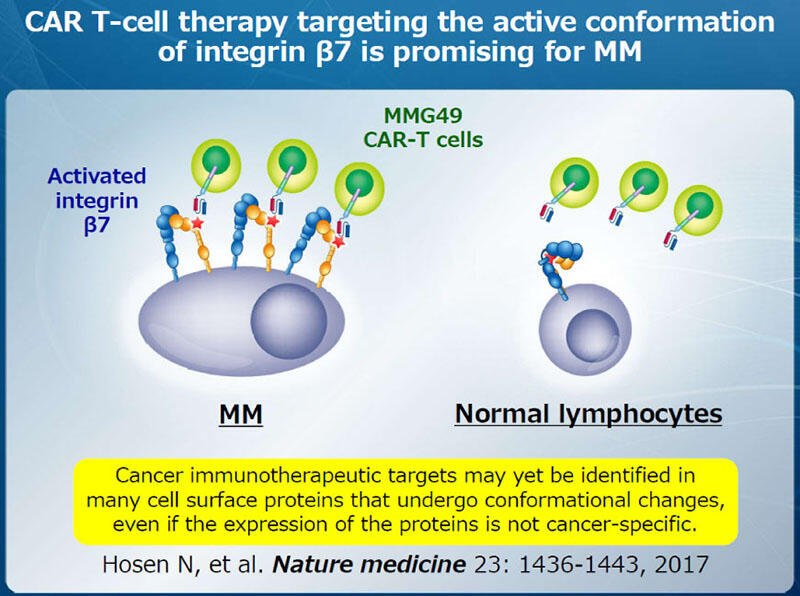
Chimeric antigen receptor (CAR)-T cell therapy is a new cellular immunotherapy targeting cancer-specific cell surface antigens. Antigen-recognition domain of a cancer-specific monoclonal antibody (mAb) is fused with a co-stimulatory molecule such as CD28 or 4-1BB and CD3 to generate CAR. CAR-T cells, which are established by transducing CAR cDNA into T cells from patients, exert high anti-tumor activity and proliferate extensively upon recognizing the cancer-specific antigens (Fig. 1). We have been working on identifying cancer-specific cell surface antigens and developing mAb therapy or CAR-T cell therapy targeting them, and recently succeeded in developing CAR-T cell therapy targeting the activated conformation of integrin β7 for multiple myeloma (Fig. 2). This result indicates that cancer immunotherapeutic targets may yet be identified in many cell surface proteins that undergo conformational changes, even if the expression of the proteins is not cancer-specific. We are now searching for similar target antigens in various types of cancers and developing CAR-T cell therapy targeting them.
Principal Investigator
Naoki Hosen Professer

Research field
hematology, cancer immunology
Research and career history
| 2003.12 | Stanford University School of Medicine, Postdoc (-2007.3) |
|---|---|
| 2007.5 | Graduate School of Medicine, Osaka University, Associate Professor (-2009.3) |
| 2009.4 | Division of Health Sciences, Graduate School of Medicine, Osaka University (-2014.3) |
| 2014.4 | Division of Health Sciences, Graduate School of Medicine, Osaka University, Associate Professor (2019.12) |
| 2020.1- | Division of Medicine, Graduate School of Medicine, Osaka University, Professor |
| 2020.11- | Immunology Frontier Research Center, Osaka University, Professor |
Prize
| 2007 | Leukemia Research Fund Young Investigator Award |
|---|---|
| 2018 | Princess Takamatsu Cancer Research Fund Research Grant |
Members
- Naoki Hosen Professer
hnaokibldon.med.osaka-u.ac.jp - Michiko Ichii Assistant Professor
michiibldon.med.osaka-u.ac.jp - Kentaro Fukushima Assistant Professor
kfukushibldon.med.osaka-u.ac.jp
Achievements
Publications
- Hosen, N., Y. Matsunaga, K. Hasegawa, H. Matsuno, Y. Nakamura, M. Makita, K. Watanabe, M. Yoshida, K. Satoh, S. Morimoto, F. Fujiki, H. Nakajima, J. Nakata, S. Nishida, A. Tsuboi, Y. Oka, M. Manabe, H. Ichihara, Y. Aoyama, A. Mugitani, T. Nakao, M. Hino, R. Uchibori, K. Ozawa, Y. Baba, S. Terakura, N. Wada, E. Morii, J. Nishimura, K. Takeda, Y. Oji, H. Sugiyama, J. Takagi, and A. Kumanogoh. The activated conformation of integrin beta7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat Med 23:1436-1443, 2017.
- Wagner, K.D., J. Cherfils-Vicini, N. Hosen, P. Hohenstein, E. Gilson, N.D. Hastie, J.F. Michiels, and N. Wagner. The Wilms' tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun 5:5852, 2014.
- Nakata, J., K. Nakano, A. Okumura, Y. Mizutani, H. Kinoshita, M. Iwai, K. Hasegawa, S. Morimoto, F. Fujiki, N. Tatsumi, H. Nakajima, Y. Nakae, S. Nishida, A. Tsuboi, Y. Oji, Y. Oka, H. Sugiyama, A. Kumanogoh, and N. Hosen. In vivo eradication of MLL/ENL leukemia cells by NK cells in the absence of adaptive immunity. Leukemia 28:1316-1325, 2014.
- Hosen, N., Y. Matsuoka, S. Kishida, J. Nakata, Y. Mizutani, K. Hasegawa, A. Mugitani, H. Ichihara, Y. Aoyama, S. Nishida, A. Tsuboi, F. Fujiki, N. Tatsumi, H. Nakajima, M. Hino, T. Kimura, K. Yata, M. Abe, Y. Oka, Y. Oji, A. Kumanogoh, and H. Sugiyama. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia 26:2135-2141, 2012.
- Hosen, N., H. Ichihara, A. Mugitani, Y. Aoyama, Y. Fukuda, S. Kishida, Y. Matsuoka, H. Nakajima, M. Kawakami, T. Yamagami, S. Fuji, H. Tamaki, T. Nakao, S. Nishida, A. Tsuboi, S. Iida, M. Hino, Y. Oka, Y. Oji, and H. Sugiyama. CD48 as a novel molecular target for antibody therapy in multiple myeloma. Br J Haematol 156:213-224, 2012.
- Martinez-Estrada, O.M., L.A. Lettice, A. Essafi, J.A. Guadix, J. Slight, V. Velecela, E. Hall, J. Reichmann, P.S. Devenney, P. Hohenstein, N. Hosen, R.E. Hill, R. Munoz-Chapuli, and N.D. Hastie. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet 42:89-93, 2010.
- Yamane, T., N. Hosen, H. Yamazaki, and I.L. Weissman. Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc Natl Acad Sci U S A 106:8953-8958, 2009.
- Hosen, N., C.Y. Park, N. Tatsumi, Y. Oji, H. Sugiyama, M. Gramatzki, A.M. Krensky, and I.L. Weissman. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A 104:11008-11013, 2007.
- Hosen, N., T. Shirakata, S. Nishida, M. Yanagihara, A. Tsuboi, M. Kawakami, Y. Oji, Y. Oka, M. Okabe, B. Tan, H. Sugiyama, and I.L. Weissman. The Wilms' tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia 21:1783-1791, 2007.
- Hosen, N., T. Yamane, M. Muijtjens, K. Pham, M.F. Clarke, and I.L. Weissman. Bmi-1-green fluorescent protein-knock-in mice reveal the dynamic regulation of bmi-1 expression in normal and leukemic hematopoietic cells. Stem Cells 25:1635-1644, 2007.
- Nishida, S., N. Hosen, T. Shirakata, K. Kanato, M. Yanagihara, S. Nakatsuka, Y. Hoshida, T. Nakazawa, Y. Harada, N. Tatsumi, A. Tsuboi, M. Kawakami, Y. Oka, Y. Oji, K. Aozasa, I. Kawase, and H. Sugiyama. AML1-ETO rapidly induces acute myeloblastic leukemia in cooperation with the Wilms tumor gene, WT1. Blood 107:3303-3312, 2006.
