News & Topics
Structural basis for semaphorin signalling through the plexin receptor. (Profs Takagi (IPR) & Kumanogoh (IFReC) in Nature)
Semaphorins and their receptor plexins constitute a pleiotropic cell-signalling system that is used in a wide variety of biological processes, and both protein families have been implicated in numerous human diseases.
The authors report the crystal structures of the semaphorin 6A (Sema6A) receptor-binding fragment and the plexin A2 (PlxnA2) ligand-binding fragment in both their pre-signalling (that is, before binding) and signalling (after complex formation) states.
Before binding, the Sema6A ectodomain was in the expected 'face-to-face' homodimer arrangement, similar to that adopted by Sema3A and Sema4D, whereas PlxnA2 was in an unexpected 'head-on' homodimer arrangement.
In contrast, the structure of the Sema6A-PlxnA2 signalling complex revealed a 2:2 heterotetramer in which the two PlxnA2 monomers dissociated from one another and docked onto the top face of the Sema6A homodimer using the same interface as the head-on homodimer, indicating that plexins undergo 'partner exchange'.
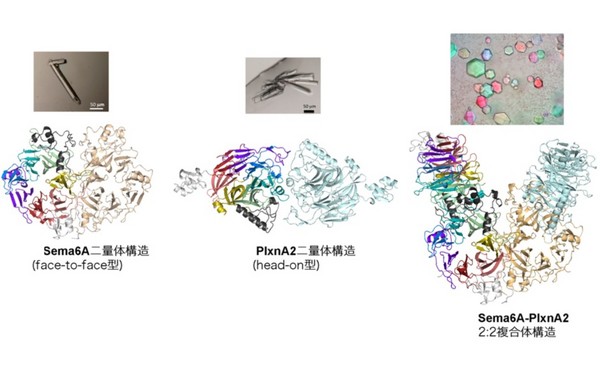 Figure: Structures of the Sema6A face-to-face homodimer (L), PlxnA2 head-on dimer (C) and Sema6A-PlxnA2 2:2 complex (R). Individual propeller blades are coloured differently in one monomer. Arrangement of the toroidal propeller domains within the structure is schematically depicted in the cartoon next to each ribbon presentation.
Figure: Structures of the Sema6A face-to-face homodimer (L), PlxnA2 head-on dimer (C) and Sema6A-PlxnA2 2:2 complex (R). Individual propeller blades are coloured differently in one monomer. Arrangement of the toroidal propeller domains within the structure is schematically depicted in the cartoon next to each ribbon presentation.
Article
Targeted Proteins Research Program
