Aging Biology
TEL +81-6-6879-4261
Overview
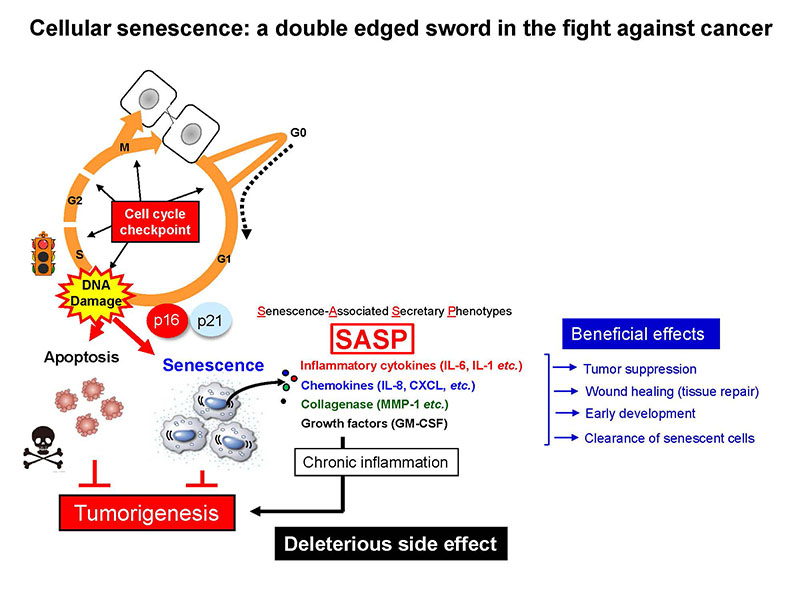
Over the last few decades, it has become apparent that oncogenic proliferative signals are coupled to a variety of growth inhibitory responses, such as the induction of apoptotic cell death or irreversible cell cycle arrest known as “cellular senescence”. Thus, both apoptosis and cellular senescence are thought to act as important tumor suppression mechanisms. Unlike apoptotic cells, however, senescent cells remain viable for long periods of time and accumulate with increasing age in various organs and tissues in vivo. Moreover, recent studies reveal that although cellular senescence initially functions as a tumor suppressive process through implementation of stable cell cycle arrest, it may eventually promote chronic inflammation through secretion of various pro-inflammatory factors called “senescence associated secretory phenotype (SASP)”. It is therefore quite possible that accumulation of senescent cells during the aging process in vivo may contribute to age-associated increases of various inflammatory disorders, such as cancer. In our laboratory, we are aiming to understand the roles and mechanisms of cellular senescence in vivo, focusing on its positive and negative roles throughout our life course. We believe that a better understanding of the molecular mechanisms involved will lead to new strategies for the prevention of aging-associated diseases such as cancer.
Principal Investigator
Eiji Hara Professor

Research field
Roles and mechanisms of cellular senescence in cancer and aging
Education history
| 1987 | B.Sc., Tokyo University of Science |
|---|---|
| 1993 | Ph.D., Tokyo University of Science |
Research and career history
| 1993.3 | Postdoctoral Fellow: University of California, Berkeley, U.S.A |
|---|---|
| 1995.1 | Postdoctoral Fellow, Imperial Cancer Research Fund Laboratories, U.K |
| 1997.1 | Lecturer, Kyoto Prefectural University of Medicine, Japan |
| 1998.9 | Group Leader, Cancer Research UK-Paterson Institute for Cancer Res., U.K |
| 2003.3 | Professor, Institute for Genome Research, University of Tokushima, Japan |
| 2008.4 | Division Chief, Cancer Institute, Japanese Foundation for Cancer Research, Japan |
| 2015.4 | Professor, Research Institute for Microbial Diseases, Osaka University, Japan |
| 2018.8 | Professor, Immunology Frontier Research Center, Osaka University, Japan |
Prize
| 2005 | Inoue Fellow, Inoue Foundation for Science |
|---|---|
| 2012 | Scientific Award, Japanese Foundation for Cancer Research |
| 2014 | JCA-Mauvernay Award, Japanese Cancer Association |
Members
- Eiji Hara Professor
eharabiken.osaka-u.ac.jp
Achievements
Publications
- Takahashi, A., Loo, T. M., Okada, R., Kamachi, F., Watanabe, Y., Wakita, M., Watanabe, S., Kawamoto, S., Miyata, K., Barber, G. N., Ohtani, N. & *Hara, E. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nature Commun., 9: 1249 (2018).
- *Okuma, A., Hanyu, A., Watanabe, S., & *Hara, E. p16INK4a and p21Cip1/Waf1 promote tumour growth by enhancing myeloid-derived suppressor cells chemotaxis. Nature Commun., 8: 2050 (2017).
- *Takasugi, M., Okada, R., Takahashi, A., Chen, D.V., Watanabe, S., & *Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nature Commun., 8: 15728 (2017).
- *Takahashi, A., Okada, R., Nagao, K., Kawamata, Y., Hanyu, A., Yoshimoto, S., Takasugi, M., Watanabe, S., Kanemaki, M.T., Obuse, C. & *Hara, E. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nature Commun., 8: 15287 (2017).
- Sato, S., Kawamata, Y., Takahashi, A., Imai, Y.,Hanyu, A., Okuma, A., Takasugi, M., Yamakoshi, K., Sorimachi, H., Kanda, H., Ishikawa, Y., Sone, S., Nishioka, Y., *Ohtani, N. & *Hara, E. Ablation of the p16INK4a tumour suppressor reverses ageing phenotypes of klotho mice. Nature Commun., 6: 7035 (2015).
- Imai, Y., Takahashi, A., Hanyu, A., Hori, S., Sato, S., Naka, K., Hirao, A., Ohtani, N. & *Hara, E. Crosstalk between the Rb Pathway and AKT Signaling Forms a Quiescence-Senescence Switch.Cell Rep., 7: 194-207 (2014)
- Yoshimoto, S., Loo, T.M., Atarashi, K., Kanda, H., Sato, S., Oyadomari, S., Iwakura, Y., Oshima, K., Morita, H., Hattori, M., Honda, K., Ishikawa, Y., *Hara, E. & Ohtani, N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature, 499: 97-101(2013)
- Takahashi, A., Imai, Y., Yamakoshi, K., Kuninaka, S., Ohtani, N., Yoshimoto, S., Hori, S., Tachibana, M., Anderton, E., Takeuchi, T., Shinkai, Y., Peters, G., Saya, H. & *Hara, E. DNA damage signaling triggers degradation of histone methyltransferases through APC/CCdh1 in senescent cells. Mol. Cell, 45: 123-131(2012)
- Yamakoshi, K., Takahashi, A., Hirota, F., Nakayama, R., Ishimaru, N., Kubo, Y., Mann, D.J., Ohmura, M., Hirao, A., Saya, H., Arase, S., Hayashi, Y., Nakao, K., Matsumoto, M., *Ohtani, N. & *Hara, E. Real-time in vivo imaging of p16INK4a reveals cross-talk with p53. J. Cell Biol., 186: 393-407 (2009)
- *Ohtani, N., Imamura, Y., Yamakoshi, K., Hirota, F., Nakayama, R., Kubo, Y., Ishimaru, N., Takahashi, A., Hirao, A., Shimizu, T., Mann, D.J., Saya, H., Hayashi, Y., Arase, S., Matsumoto, M., Nakao, K. & Hara, E. Visualizing the dynamics of p21Waf1/Cip1 cyclin-dependent kinase inhibitor expression in living animals Proc. Natl. Acad. Sci. USA, 104: 15034-15039 (2007)
- Takahashi, A., Ohtani, N., Yamakoshi, K., Iida, S., Tahara, H.,Nakayama, K., Nakayama, K.I., Ide, T., Saya, H. & *Hara, E. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce rreversible cellular senescence Nature Cell Biol., 8: 1291-1297 (2006)
- Maehara, K., Yamakoshi, K., Ohtani, N., Kubo, Y., Takahashi, A., Arase, S., Jones, N. & *Hara, E. Reduction of total E2F/DP activity induces senescence-like cell cycle arrest in cancer cells lacking functional pRB and p53. J. Cell Biol., 167: 553-560 (2005)
- Ohtani, N., Brennan, P., Gaubatz, S., Sanij, E., Hertzong, P., Wolvetang, E., Ghysdael, J., Rowe, M. & *Hara, E. Epstein-Barr virus LMP1 blocks p16 INK4a-RB-pathway by promoting nuclear export of E2F4/5. J. Cell Biol., 162: 173-183 (2003)
- Ohtani, N., Zebedee, Z., Huot, T.J.G., Stinson, J.A., Sugimoto, M., Ohashi, Y., Sharrrocks, A.D., Peters, G. & *Hara, E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature, 409: 1067-1070 (2001).
- Sugimoto, M., Nakamura, T., Ohtani, N., Hampson, L., Hampson, I.N., Shimamoto, A., Furuichi, Y., Okumura, K., Niwa, S., Taya Y. & *Hara, E. Regulation of CDK4 activity by a novel CDK4 binding protein, p34SEI-1. Genes & Dev., 13: 3027-3033 (1999).
