Innate immune systems
TEL +81-6-6879-3820
Overview
The study of ILC2 in allergy and immune diseases
ILC2 (originally named “natural helper cells”), found in fat associated lymphoid clusters (FALC), do not express antigen-specific receptors found on many other immune cells. Nevertheless, they respond rapidly to the IL-25 and IL-33 secreted by damaged epithelial cells by secreting large amounts of IL-5 and IL-13, which leads to parasite elimination and induction of allergic responses .
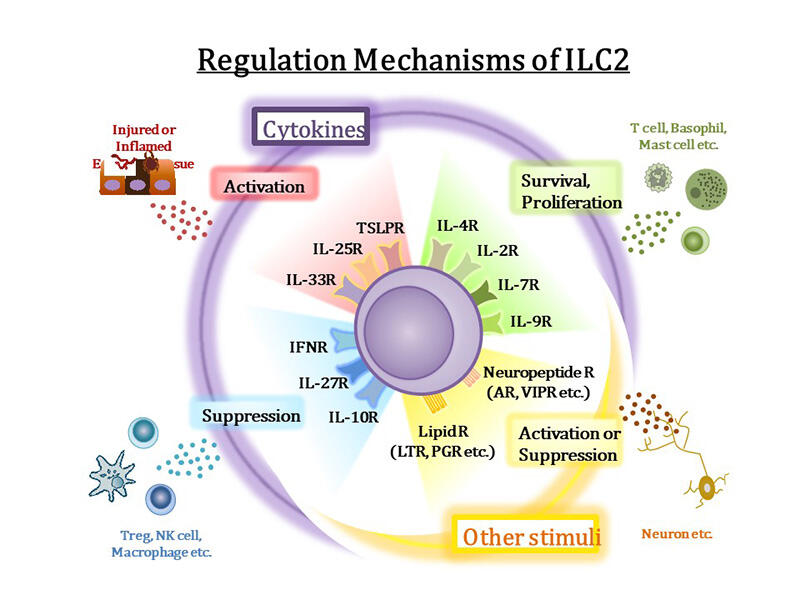
The mechanism for activation of ILC2 differs from that of T helper 2 (Th2) cells, which also produce type 2 cytokines: Th2 cells do so in an antigen-specific manner, whereas ILC2 do not.
The discovery of ILC2 confirmed the existence of the ILC family, which comprises lymphocytes with helper activity in the innate system. There are now three categories of ILCs. ILC1 produce IFNγ and have antiviral and antitumor immunity. As mentioned above, ILC2 produce IL-5 and IL-13 and are associated with parasitic responses and allergies. Finally, ILC3 secrete IL-17 and IL-22 and are associated with autoimmune diseases.
Accordingly, the ILC family as a whole differ from Th cells in a number of ways. Th cells take several days to differentiate, while ILCs are present in normal tissues as mature lymphocytes. Thus, besides their role in immune responses, ILCs are believed to contribute to tissue homeostasis. ILC2, in particular, are found in tissues throughout the body including the lungs, bone, brain, muscle, skin and others. In addition to allergic reactions, they are associated with chronic inflammation in obesity and fibrosis, and play a role in rheumatoid arthritis, an autoimmune disease. Furthermore, they have been reported to interact with non-immune cells, promote the proliferation of tissue stem cells and epithelial cells for regeneration, and localize around nerves to regulate neurotransmitters.
Much of the above research has focused on mouse models, and many efforts are underway to confirm that the same properties hold true in humans. Genetic polymorphism studies have suggested ILC2-related genes such as IL-33 are associated with disease and ILC2 is being investigated as a new clinical target .
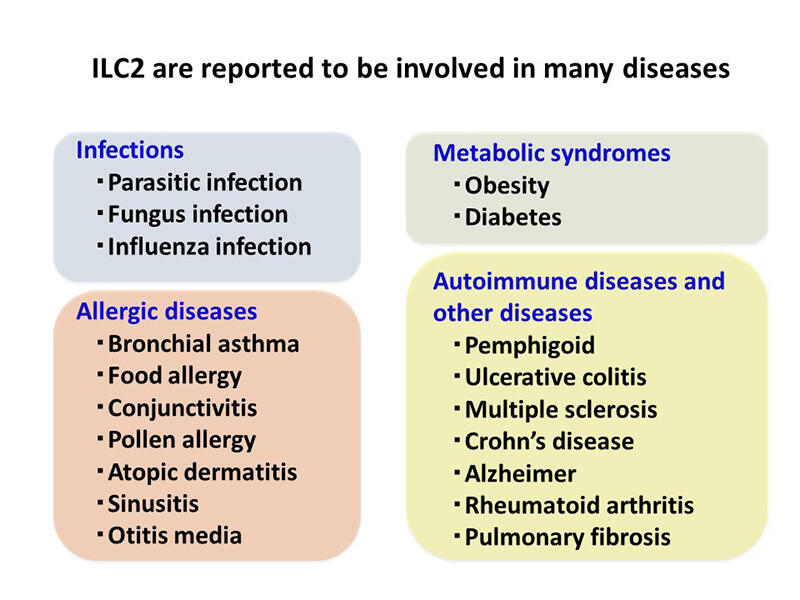
With all the above points in mind, our laboratory is conducting comprehensive analysis of ILC2, including investigation of the mechanisms for differentiation, signaling and activation and also partner cell types. The expectation is that this research will lead to new models and therapies for related immune diseases such as allergies, fibrosis, and metabolic diseases.
Principal Investigator
Kazuyo Moro Professor

Research field
Innate immune systems
Education history
| 2003 | DDS, Nihon University, Japan |
|---|---|
| 2010 | PhD, Keio University, Japan |
Research and career history
| 2003 | Research Assistant, COE, Keio University School of Medicine, Japan |
|---|---|
| 2007 | Assistant Professor, Keio University School of Medicine, Japan |
| 2008 | Assistant Professor, Global COE, Keio University School of Medicine, Japan |
| 2011 | Research Fellow, PRESTO, JST, Japan |
| 2011 | Assistant Professor, Immunology, Keio University School of Medicine, Japan |
| 2012 | Sub Team Leader, RIKEN RCAI, Yokohama, Japan |
| 2013 | Senior Research Fellow, RIKEN IMS, Yokohama, Japan |
| 2013 | Guest Associate Professor, Yokohama City University, Japan |
| 2015 | Team Leader, Innate Immune System, RIKEN IMS, Yokohama, Japan |
| 2016 | Guest Professor, Yokohama City University, Japan |
| 2016 | Program Officer, AMED, Research Project for Intractable diseases, Japan |
| 2019 | Professor, Osaka University Graduate School of Medicine, Japan |
Prize
| 2011 | 27th Inoue Research Award for Young Scientists |
|---|---|
| 2016 | 11th Japanese Society of Immunology (JSI) Young Investigator Award |
| 2017 | 13th Japan Society for the Promotion of Science (JSPS) Prize |
| 2017 | 13th Japan Academy Medal |
| 2018 | 8th Nagase Award |
Members
-
Kazuyo Moro Professor
moroilc.med.osaka-u.ac.jp
Achievements
Publications
-
Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S; Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463 (7280)540-544 (2010)
-
Furusawa J, Moro K, Motomura Y, Okamoto K, Zhu J, Takayanagi H, Kubo M, Koyasu S; Critical role of p38 and GATA3 in natural helper cell function. Journal of immunology. 191 (4)1818-1826 (2013)
-
Kabata H, Moro K (equal contribution), Fukunaga K, Suzuki Y, Miyata J, Masaki K, Betsuyaku T, Koyasu S, Asano K; Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 42675 (2013)
-
Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, Kallies A; The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 16 (3)276-285 (2015)
-
Moro K, Ealey KN, Kabata H, Koyasu S; Isolation and analysis of group 2 innate lymphoid cells in mice. Nature protocols. 10 (5)792-806 (2015)
-
Moro K, Koyasu S; Innate lymphoid cells, possible interaction with microbiota. Seminars in immunopathology. 37 (1)27-37 (2015)
-
Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T, Koyasu S; Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 17 (1)76-86 (2016)
-
Ealey KN, Moro K, Koyasu S; Are ILC2s Jekyll and Hyde in airway inflammation? Immunological reviews. 278 (1)207-218 (2017)
-
Koga S, Hozumi K, Hirano KI, Yazawa M, Terooatea T, Minoda A, Nagasawa T, Koyasu S, Moro K; Peripheral PDGFRalpha(+)gp38(+) mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med. 215 (6)1609-1626 (2018)
-
Kabata H, Moro K, Koyasu S; The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunological reviews. 286 (1)37-52 (2018)
