Lymphocyte Differentiation
TEL +81-6-6879-4457
FAX +81-6-6879-4457
Overview
Tracing the fate determination mechanism of B lymphocytes in immunity
Living organisms such as human are equipped with immune systems to defend themselves against foreign invaders such as bacteria and viruses. The core function of this immune system is executed by B lymphocytes, which recognize foreign bodies and eliminate them by producing antibodies. B lymphocytes can produce an unlimited number of different antibodies against a diverse array of foreign bodies by utilizing random rearrangement of gene segments encoding antibodies (immunoglobulin genes). However, this random genetic change can inevitably make a risk of producing antibodies that attack the body itself; this phenomenon is manifested as so-called "autoimmune diseases".
It is our biggest issue to clarify what sorts of mechanisms make B lymphocytes to differentiate and proliferate, to select cells that recognize foreign bodies (good cells) or our body itself (bad cells), to eliminate bad cells and maintain good cells and to differentiate good cells into plasma cells, thereby leading to production of only good antibodies. Of course, understanding these mechanisms is able to lead to development of more-efficient therapies to cure immune disease such as allergy and autoimmune diseases.
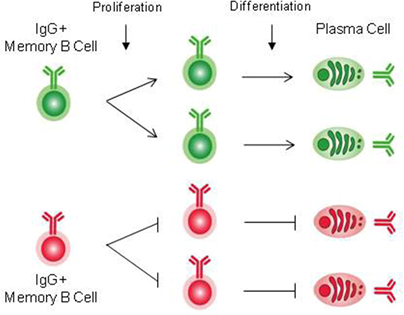
Function of PLCγ2 in humoral memory responses
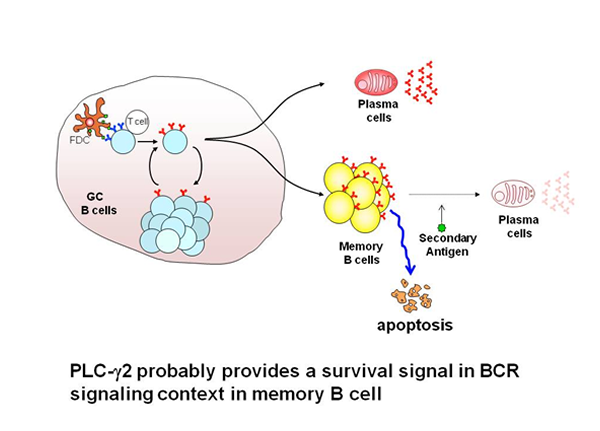
Humoral memory is characterized by recall immune responses, which are more rapid than the primary response, and by the production of higher serum titers of antigen-specific antibodies, mostly of the IgG isotype. The prevailing view is that antigen-specific B cells are maintained as a pool of memory B cells after clonal expansion during the primary immune responses. Most memory B cells have been thought to originate from the germinal center (GC) reaction. In the GC, the combined processes of somatic hypermutation and selection based on the affinity of the BCR for the antigen, are responsible for the generation of high-affinity antibody variants which ultimately differentiate into long-lived plasma cells or long-lived memory B cells. In the GC reaction, de novo generated antigen-specific memory B cells are thought to acquire intrinsically different traits from their naive predecessors, accounting for more rapid and heightened secondary responses. Thus, understanding the mechanisms by which memory B cells are generated and are maintained as well as intrinsic functional differences existing between naive and memory B cells are of fundamental interest to reveal the basis of immunological memory. To ask whether the establishment and maintenance of memory B cells require BCR signaling, we have focused on the function of PLCγ2, as this enzyme has been established as an important signaling component of the BCR signaling pathway. We show that PLCγ2 is required for the efficient generation and maintenance of memory B cells, probably through the delivery of a pro-survival signal.
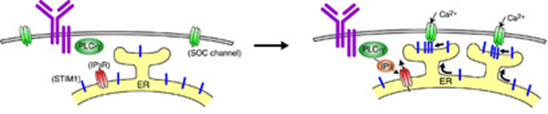
Mechanism of calcium regulation
Calcium ions are used by various cell types, including immune cells, bone cells, and neurons, to regulate their physiological response. Typically, calcium is sourced from stores in an intracellular compartment known as the ER. When these stores are depleted, the cell must bring in calcium from the extracellular milieu. Important in this process is a protein in the ER, called STIM1. Baba et al. have shown for the first time that STIM1 normally resides in a specialized sub-compartment of the ER. Also, while confirming earlier studies demonstrating that calcium depletion in the ER stores stimulates STIM1 to translocate to regions near the cell surface, they have shown that insertion of STIM1 into the plasma membrane (PM) is not necessary for opening calcium channels in the PM.
The above results were obtained by using a B cell culture system. Now, Baba et al. have extended the conclusion that STIM1 functions as a critical regulator for inducing calcium influx to primary mast cells, by analyzing STIM1knock-out mice. Indeed, STIM1-deficient fetal liver-derived mast cells had impaired Ca2+ influx mediated by FcεRI and activation of the transcription factors NF-κB and NFAT. Mast cells lacking STIM1 also had much less degranulation and cytokine production after FcεRI stimulation. In addition, alterations in STIM1 expression affected the sensitivity of IgE-mediated immediate-phase anaphylactic responses in vivo. Thus, STIM1 has turned out to be key in promoting the Ca2+ influx that is essential for FcεRI-mediated primary mast cell activation and anaphylaxis.
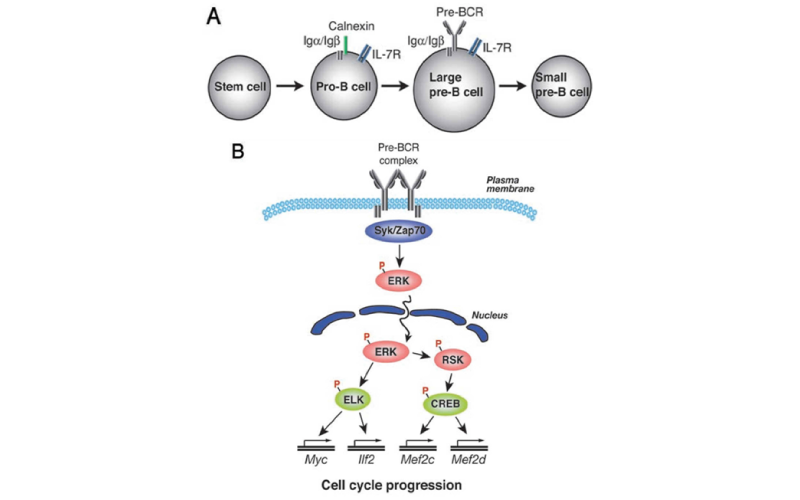
Function of Ras/Erk signaling in B cell biology
We demonstrated that BCR signaling required RasGRP3, whereas epidermal growth factor receptor signaling was found to be dependent on Sos1 and Sos2. Further, we demonstrated that BCR-induced recruitment of RasGRP3 to the cell membrane and subsequent Ras activation were significantly attenuated in PLC-γ2-deficient B cells, suggesting that PLC-γ2 is involved in RasGRP3 localization and, subsequently, Ras activation. In terms of the RasGRP3 activation mechanism, in addition to recruitment, enzymatic activation of RasGRP3 through phosphorylation is required for activation. Following BCR cross-linking, RasGPR3 undergoes phosphorylation at Thr-133 which results in its activation.
BCR-mediated Erk activation takes place downstream of Ras. Activation of ERK has been implicated in proliferation as well as differentiation in a variety of cell types. However, there is no direct evidence for its role in B cell development and activation. Thus, Sanjo et al., by using B-cell-specific ERK2-targeted mice, have shown that in T- cell-dependent immune responses, ERK2 is required to generate efficient IgG production. In its absence, the proportion of antigen-specific IgG1-bearing cells and subsequent number of IgG1 antibody-secreting cells were decreased, despite apparently unimpaired class-switching. To further analyze the function of ERK1 and ERK2 in B cell development, we have generated mice deficient in both ERK1 and ERK2. Deletion of both of these genes is associated with defective pre-BCR-mediated cell expansion as well as a block in the transition of pro-B to pre-B cells. Phosphorylation of transcription factors ELK1 and CREB is mediated by ERK, and a dominant-negative mutation in the ERK-mediated phosphorylation sites of ELK1 or CREB suppressed pre-BCR-mediated cell expansion as well as expression of genes including Myc, which is involved in cell-cycle progression. Together, these results identify a crucial role for ERK kinases in regulating B cell development by initiating a transcriptional regulatory network and thereby pre-BCR-mediated cell expansion.
Members
- Tomohiro Kurosaki Guest Professor
kurosakiifrec.osaka-u.ac.jp - Takeshi Inoue Guest Professor
inoueifrec.osaka-u.ac.jp
Achievements
Publications
- Inoue, T., Morita, M., Hijikata, A., Fukuda-Yuzawa, Y., Adachi, S., Isono, K., Ikawa, T., Kawamoto, H., Koseki, H., Natsume, T., Fukao, T., Ohara, O., Yamamoto, T., Kurosaki, T.
CNOT3 contributes to early B cell development by controlling Igh rearrangement and p53 mRNA stability.
J. Exp. Med. pii: jem.20150384 - Onishi, M., Ozasa, K., Kobiyama, K., Ohata, K., Kitano, M., Taniguchi, K., Homma, T., Kobayashi, M., Sato, A., Katakai, Y., Yasutomi, Y., Wijaya, E., Igarashi, Y., Nakatsu, N., Ise, W., Inoue, T., Yamada, H., Vandenbon, A., Standley, D.M., Kurosaki, T., Coban, C., Aoshi, T., Kuroda, E., Ishii, K.J.
Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen.
J Immunol. 15;194:2673-82. - Uebelhart, R., Hug, E., Bach, MP., Wossning, T., Duhren-von Minden, M., Horn, AHC., Kometani, K., Kurosaki, T., Sticht, H., Reth, M., Jumaa, H.
Responsiveness of B cells is regulated by the hinge region of IgD.
Nat Immunol. 16:534-43. 2015 - Baba, Y., Matsumoto, M., Kurosaki, T.
Signals controlling the development and activity of regulatory B-lineage cells.
Int. Immunol. pii: dxv027 - Kurosaki, T., Kometani, K., Ise, W.
Memory B cells.
Nat Rev Immunol. 15:149-59. 2015 - Kometani, K., Kurosaki, T.
Differentiation and maintenance of long-lived plasma cells.
Curr Opin Immunol.33C:64-69. 2015 - Uebelhart, R., Hug, E., Bach, MP., Wossning, T., Duhren-von Minden, M., Horn, AHC., Kometani, K., Kurosaki, T., Sticht, H., Reth, M., Jumaa, H. Structural Differences between IgD and IgM control B cell tolerance and the generation of innate-like B1 cells.
Nat. Immunol. in press - Baba, Y., Matsumoto, M., Kurosaki, T.
Calcium signaling in B cells: regulation of cytosolic Ca2+ increase and its sensor molecules, STIM1 and STIM2.
Mol Immunol.62:339-43. 2014 - Wing, J.B, Ise, W., Kurosaki, T., Sakaguchi, S.
Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4.
Immunity41:1013-25. 2014 - Tanaka, S., Tanaka, K., Magnusson, F., Chung, Y., Martinez, G.J., Wang, Y.H., Nurieva, R.I., Kurosaki, T., Dong, C.
CCAAT/Enhancer-Binding Protein α Negatively Regulates IFN-g Expression in T Cells.
J Immunol. 193:6152-60 - Hendron, E., Wang, X., Zhou, Y., Cai, X., Goto, J.I., Mikoshiba, K., Baba, Y.,Kurosaki, T., Wang, Y., Gill, D.L.
The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program.
Cell Calcium. 56:482-492. - Itoh-Nakadai, A., Hikota, R., Muto, A., Kometani, K., Watanabe-Matsui, M., Sato, Y., Kobayashi, M., Nakamura, A., Miura, Y., Yano, Y., Tashiro, S., Sun, J., Ikawa. T., Ochiai, K., Kurosaki, T., Igarashi, K.
The transcription repressors Bach2 and Bach1 promote B cell development by repressing the myeloid program.
Nat Immunol. 15:1171-80. - Matsumoto, M., Baba, A., Yokota, T., Nishikawa, H., Ohkawa, Y., Kayama, H., Kallies, A., Nutt, S.L., Sakaguchi, S., Takeda, K., Kurosaki, T., Baba, Y.
Interleukin-10-Producing Plasmablasts Exert Regulatory Function in Autoimmune Inflammation.
Immunity 41:1040-51 - Schimmack, G., Eitelhuber, A.C., Vincendeau, M., Demski, K., Shinohara, H., Kurosaki, T., Krappmann, D.
AIP augments CARMA1-BCL10-MALT1 complex formation to facilitate NF-κB signaling upon T cell activation.
Cell Commun Signal. 12:49 111:11792-7 - Ise, W., Inoue, T., McLachlan, J.B., Kometani, K., Kubo, M., Okada, T.Kurosaki, T.
Memory B cells contribute to rapid Bcl6 expression by memory TFHcells.
Proc. Natl. Acad. U.S.A. 111:11792-7 - Furukawa, Y., Teraguchi, S., Ikegami, T., Dagliyan, O., Jin, L., Hall, D., Dokholyan, N.V., Namba, K., Akira, S., Kurosaki, T., Baba, Y., Standley, D.M.
Intrinsic disorder mediates cooperative signal transduction in STIM1.
J Mol Biol. 426:2082-97, 2014 - Shinohara, H., Behar, M., Inoue, K., Hiroshima, M., Yasuda, T., Nagashima, T., Kimura, S., Sanjo, H., Maeda, S., Yumoto, N., Ki, Sewon, Akira, S., Sako, Y., *Hoffmann, A., *Kurosaki, T., *Okada-Hatakeyama, M. (*co-corresponding authors)
Positive Feedback Within a Kinase Signaling Complex Functions as a Switch Mechanism for NF-κB Activation.
Science 344:760-764, 2014 - Hartmann. J., Karl, R.M., Alexander, R.P., Adelsberger, H., Brill, M.S., Ruhlmann, C., Ansel, A., Sakimura, K., Baba. Y., Kurosaki, T., Misgeld, T., Konnerth, A.
STIM1 Controls Neuronal Ca2+ Signaling, mGluR1-Dependent Synaptic Transmission, and Cerebellar Motor Behavior.
Neuron. 82:635-44, 2014 - Yasukawa. S., Miyazaki, Y., Yoshii, C., Nakaya, M., Ozaki, N., Toda, S., Kuroda, E., Ishibashi, K., Yasuda, T., Natsuaki, Y., Mi-ichi, F., Iizasa, E., Nakahara, T., Yamazaki, M., Kabashima, K., Iwakura, Y., Takai, T., Saito, T., Kurosaki, T., Malissen, B., Ohno, N., Furue, M., Yoshida, H., and Hara, H.
An ITAM-Syk-CARD9 signaling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells.
Nature Comm. 5:3755, 2014 - Masahata, K., Umemoto, E., Kayama, H., Kotani, M., Nakamura, S., Kurakawa, T., Kikuta, J., Gotoh, K., Motooka, D., Sato, S., Higuchi, T., Baba, Y., Kurosaki, T., Kinoshita, M., Shimada, Y., Kimura, T., Okumura, R., Takeda, A., Tajima, M., Yoshie, O., Fukuzawa, M., Kiyono, H., Fagarasan, S., Iida, T., Ishii, M., Takeda, K.
Generation of colonic IgA-secreting cells in the cecal patch.
Nature Comm. 5:3704, 2014 - Kuwahara, M., Suzuki, J., Tofukuji, S., Yamada, T., Kanoh, M., Matsumoto, A., Maruyama, S., Kometani, K., Kurosaki, T., Ohara, O., Nakayama, T., Yamashita, M.
The Menin-Bach2 axis is critical for regulating CD4 T cell senescence and cytokine homeostasis.
Nature Comm. 5:3555, 2014 - Zhang, H., Clemens, R.A., Liu, F., Hu, Y., Baba, Y., Theodore, P., Kurosaki, T., and Lowell, C.A.
The STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense.
Blood 123:2238-49, 2014 - Lorès P, Vernet N, Kurosaki T, Van de Putte T, Huylebroeck D, Hikida M, Gacon G, Tourè A.
The STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense.
Dev Biol.386, 419-427, 2014 - Capietto, A.H., Kim, S., Sanford, D.E., Liehan, D.C., Hikida, M., Kurosaki, T., Novack, D.V. and Faccio, R.
Downregulation of PLCgamma2-beta-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer.
J. Exp. Med. 210, 2257-2271, 2013 - Sasaki, Y., Sano, S., Nakahara, M., Murata, S., Kometani, K., Aiba, Y., Sakamoto, S., Watanabe, Y., Tanaka, K., Kurosaki, T., and Iwai, K.
Defective immune responses in mice lacking LUBAC-mediated linear ubiquitination in B cells.
EMBO J. 32, 2463-76, 2013 - Dieterle, A.M., Bohler, P., Keppeler, H., Alers, S., Berleth, N., Driesen, S., Hieke, N., Pietkiewicz, S., Loffler, A.S., Peter, C., Gray, A., Leslie, N.R., Shinohara, H., Kurosaki, T., Engelke, M., Wienands, J., Bonin, M., Wesselborg, S., and Stork, B.
PDK1 controls upstream PI3K expression and PIP3 generation.
Oncogene 2013 - Kometani, K., Nakagawa, R., Shinnakasu, R., Kaji, T., Rybouchkin, A., Moriyama, S., Furukawa, K., Koseki, H., Takemori, T. and Kurosaki, T.
Repression of the Transcription Factor Bach2 Contributes to Predisposition of IgG1 Memory B Cells toward Plasma Cell Differentiation.
Immunity 39, 136-147, 2013 - Tsukumo, S., Unno, M., Muto, A., Takeuchi, A., Kometani, K., Kurosaki, T., Igarashi, K., Saito, T.
Bach2 maintains T cells in a naive state by suppressing effector memory-related genes.
Proc. Natl. Acad. Sci. U.S.A. 110:10735-40, 2013
