News & Topics
Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. (Associate Prof. Masaru Ishii in Nature)
Osteoclasts are bone-resorbing multinuclear giant cells that differentiate from mononuclear macrophage/monocyte-lineage hematopoietic precursors. They play critical roles not only in normal bone homeostasis (called "bone remodeling"), but also in the pathogenesis of bone destructive disorders such as rheumatoid arthritis and postmenopausal osteoporosis. A major focus of research in this field so far has been mainly on gene regulation by osteoclastogenic cytokines, such as RANKL and TNFa, both of which have been well documented to contribute to osteoclast terminal differentiation. On the other hand, a key question that has been less well studied is the process of recruitment of osteoclast precursors to the bone surface where they undergo cell fusion to form the fully differentiated multinucleate cells that actually mediate bone resorption.
To address this question, the authors have developed a novel method for direct in situ visualization of cell behavior using intravital 2 photon imaging. The application of this new imaging technologies add a critical dynamic dimension to the analysis, transforming speculations derived from indirect observations into conclusions based on direct evidence. The major finding of my current study is that sphingosine-1-phosphate (S1P), a lipid mediator (chemokine) enriched in blood, controls the movement of osteoclast precursors between the blood and the bone surface. Moreover, the authors proposed a novel therapeutics against bone-resorptive disorders with FTY720, an S1P agonist being studied clinically as an immunosuppressant.
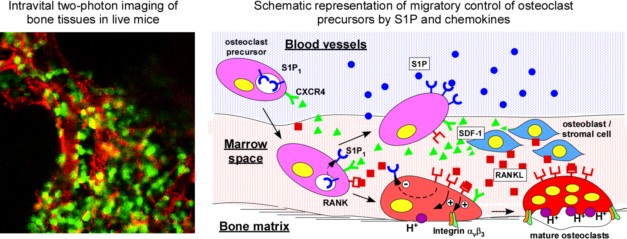
The authors believe this work, with its use of novel methods for assessing cell migration in situ, its documentation of a previously unknown molecular mechanism controlling osteoclast precursor migration demonstrated using an outstanding imaging technique, will pioneer a conceptually new research field in this field, and would facilitate the development of novel therapeutics against bone-resorptive disorders with a new medicinal property.
This work was supported by the Intramural Research Program, NIAID, NIH, and partly by the International Human Frontier Science Program.
Article
Contact:
Masaru IshiiLaboratory of Biological Imaging,
Immunology Frontier Research Center, Osaka University
3-1 Yamada-oka, Suita, Osaka 565-0871, Japan
Tel: +81-6-6879-8333, Fax: +81-6-6879-8332
mishii@ifrec.osaka-u.ac.jp
