News & Topics
GPI Glycan Remodeling by PGAP5 Regulates Transport of GPI-Anchored Proteins from the ER to the Golgi. (Prof. Kinoshita in Cell)
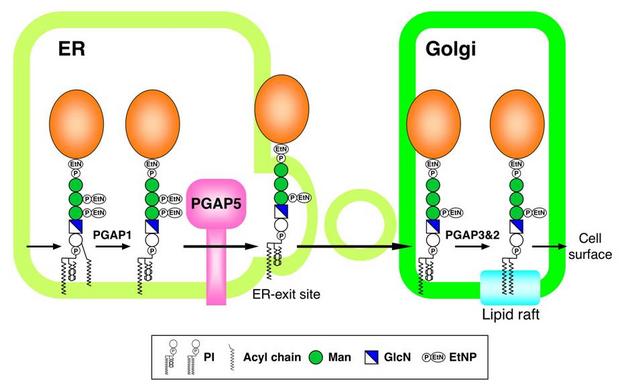
Many eukaryotic proteins are attached to the cell surface via glycosylphosphatidylinositol (GPI) anchors. How GPI-anchored proteins (GPI-APs) are trafficked from the endoplasmic reticulum (ER) to the cell surface is poorly understood, but the GPI moiety has been postulated to function as a signal for sorting and transport.
Here, we established mutant cells that were selectively defective in transport of GPI-APs from the ER to the Golgi. We identified a responsible gene, designated PGAP5 (post- GPI-attachment to proteins 5). PGAP5 belongs to a dimetal-containing phosphoesterase family and catalyzed the remodeling of the glycan moiety on GPI-APs. PGAP5 catalytic activity is a prerequisite for the efficient exit of GPI-APs from the ER.
Our data demonstrate that GPI glycan acts as an ERexit signal and suggest that glycan remodeling mediated by PGAP5 regulates GPI-AP transport in the early secretory pathway.
Article
Contact:
Taroh KINOSHITALaboratory of Immunoglycobiology,
WPI Immunology Frontier Research Center
Integrated Life Science Building, Osaka University
3-1 Yamada-oka, Suita City, Osaka 565-0871, Japan
Tel: +81-6-6879-8328, Fax: +81-6-6875-5233
tkinoshi@biken.osaka-u.ac.jp
