News & Topics
The tertiary structure of the human Xkr8-Basigin complex that scrambles phospholipids at plasma membranes (Nagata G, in Nat Struct Mol Biol)
The research group of Takaharu Sakuragi and Shigekazu Nagata (Biochemistry & Immunology, IFReC) combined cryo-EM and X-ray crystallography to investigate its structure at an overall resolution of 3.8 Å. The structure of Xkr8-Basigin revealed by them provides insights into the molecular mechanisms underlying phospholipid scrambling.
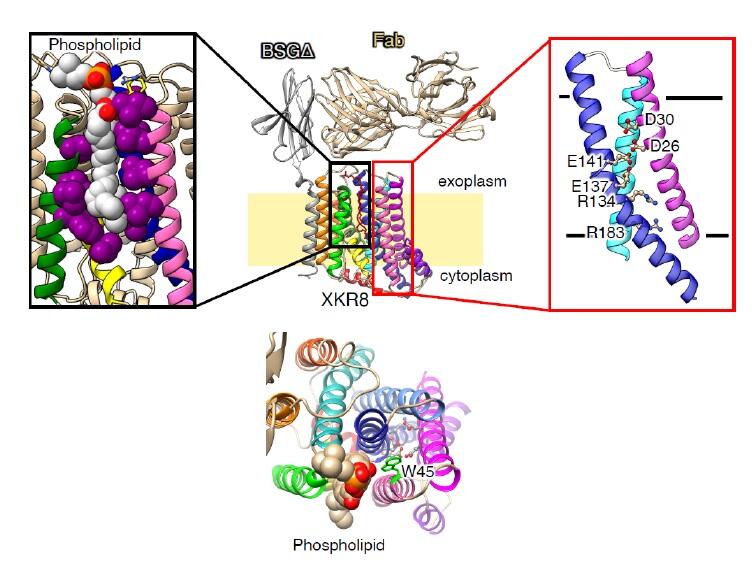 Figure: (L) Front view of the hXkr8-hBSGΔ complex. Hydrophobic residues surrounding PtdCho are in magenta spheres. PtdCho is in the silver sphere with the colored element. (R)Close-up side and top views of charged amino acids (Glu, Asp and Arg) in the α1, α4 and α5 helices. (Bottom) Extracellular view of the Xkr8 molecule. The Trp residues (green), which act as gatekeepers, are located at the boundary between phospholipids and the phospholipid transit region (pore).
Figure: (L) Front view of the hXkr8-hBSGΔ complex. Hydrophobic residues surrounding PtdCho are in magenta spheres. PtdCho is in the silver sphere with the colored element. (R)Close-up side and top views of charged amino acids (Glu, Asp and Arg) in the α1, α4 and α5 helices. (Bottom) Extracellular view of the Xkr8 molecule. The Trp residues (green), which act as gatekeepers, are located at the boundary between phospholipids and the phospholipid transit region (pore).
Commentary (PDF)
Article (External Link)
Contact:

Shigekazu Nagata (Biochemistry & Immunology)
![]() +81-6-6879-4953
+81-6-6879-4953![]() snagataifrec.osaka-u.ac.jp
snagataifrec.osaka-u.ac.jp
